‘Bionic eye’ approved by FDA
Daily News Article — Posted on February 19, 2013
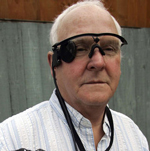 (WJLA ABCNews) AP – Patients who have lost their sight due to a rare disorder may be able to regain some vision using a new implantable device that takes the place of damaged cells inside the eye.
(WJLA ABCNews) AP – Patients who have lost their sight due to a rare disorder may be able to regain some vision using a new implantable device that takes the place of damaged cells inside the eye.
The Food and Drug Administration (FDA) on Thursday approved the Argus II Retinal Prosthesis System as the first treatment for an inherited disorder that causes the breakdown of cells in the retina, a membrane inside the eye.
…The device was previously approved in Europe in late 2011.
The system includes a small video camera and transmitter mounted on a pair of glasses. Images from the camera are processed into electronic data that is wirelessly transmitted to electrodes implanted into the patient’s retina.
The FDA says that while the device will not…restore patients’ vision, “it may allow them to detect light and dark in the environment,” which could help them perform daily tasks.
The FDA approved the device from Second Sight Medical Products for patients 25 years and older who have advanced retinitis pigmentosa (RP). Starting in their twenties, people with the disease slowly lose vision as the light-sensitive cells that line the retina deteriorate. Over a period of decades the condition eventually leads to blindness.
“It’s like looking down a tunnel that gradually narrows until it disappears entirely,” said Dr. Robert Greenberg, CEO and founder of Second Sight. “What we’re doing is reopening the window that had closed on them.”
Greenberg first proposed the technology for the Argus device as a doctoral student at Johns Hopkins University’s medical school about 20 years ago. He founded Second Sight to develop the technology in 1998.
About 100,000 people in the U.S. have retinitis pigmentosa, though the FDA estimates fewer than 4,000 will initially receive the device under its currently approved indication. Patients must have little to no light perception in both eyes but a prior history of being able to make out basic shapes and forms. They must also have signs of at least some remaining retinal function.
Results from a study of 30 patients with the condition showed that most were able to perform daily activities better with the implant than without it. Activities included navigating sidewalks and curbs, matching different color socks and recognizing large words or sentences.
Second Sight hopes to eventually win approval to treat a wide variety of vision disorders, including macular degeneration, the leading cause of blindness in developed countries.
Research and development of the Argus II was supported by $100 million in grant funding from (three federal government agencies) – the National Institutes of Health, the National Science Foundation and the Department of Energy (and an additional $100M in private investments).
Second Sight Medical Products, Inc. is a privately held company based in Sylmar, Calif.
ABC News local WJLA. All Rights Reserved. (From an Associated Press report.) Reprinted here for educational purposes only. May not be reproduced on other websites without permission from ABC News/WJLA. Visit the website at stlouis.cbslocal.com. (Note: This article was published at WJLA on Feb. 15, 2013.)
Questions
NOTE TO STUDENTS: Read the "Background" below the questions and watch the video under "Resources" to gain a clearer understanding of RT and the Argus II.
1. a) What is retinitis pigmentosa? Be specific.
b) How many people in the U.S. are affected with this disease? What percent of the U.S. population is this?
2. What does the Argus II do? How does it work?
3. Who is eligible to try this device?
4. How has the Argus II helped the people who have been given the device?
5. a) Who developed the Argus II? When did he do so?
b) How do doctors like Dr. Greenberg, and Dr. Andrew Lee (lead surgeon in the successful double arm transplant surgery on injured soldier Brendan Marrocco) inspire you?
Background
FDA Approval of the Argus II:
Before approving it, the FDA says it reviewed data from a clinical study of 30 patients with RP who were equipped with the Argus II and monitored for at least two years after receiving the implant.
After implant surgery, 19 of the 30 participants reported no adverse events related to the surgery or device, while 11 reported a total of 23 adverse events, including inflammation, retinal detachment, and the opening of a wound along the surgical suture.
The FDA is categorizing the system as a humanitarian use device, meaning there is a "reasonable assurance" that the device is safe and its "probable benefit outweighs the risk of illness or injury."
Most participants reported that they were able to perform basic activities better with the Argus II than without it, ranging from detecting the direction of a motion, detecting street curbs, walking on a sidwalk without stepping off, and matching black, gray, and white socks. (from a cnet news article)
About Retinitis Pigmentosa (RP):
RP, an inherited retinal degenerative disease that often results in nearly complete blindness, affects roughly 100,000 Americans. The Argus II System is intended to help the worst-affected RP patients, and this approval was made under a Humanitarian Device Exemption intended to expedite market introduction of technologies intended to treat smaller, underserved patient populations. (from a yahoofinance article)
RP is a rare genetic eye condition that damages the light-sensitive cells that line the retina. In a healthy eye, these cells change light rays into electrical impulses and send them through the optic nerve to the area of the brain that assembles the impulses into an image. In people with RP, the light-sensitive cells slowly degenerate resulting in gradual loss of side vision and night vision, and later of central vision. The condition can lead to blindness. (from a washingtonexaminer article)
Who is eligible for the Argus II implant:
The FDA has just approved the the first implanted device to treat adults, age 25 years or older, with severe to profound retinitis pigmentosa (RP) who have bare light perception (can perceive light, but not the direction from which it is coming) or no light perception in both eyes, evidence of intact inner layer retina function, and a previous history of the ability to see forms. (from the washingtonexaminer article)
Why the Argus II is such good news:
- With the artificial retina or retinal prosthesis, a blind person cannot see in the conventional sense but can identify outlines and boundaries of objects, especially when there is contrast between light and dark - fireworks against a night sky or black socks mixed with white ones in the laundry.
- "Without the system, I wouldn't be able to see anything at all, and if you were in front of me and you moved left and right, I'm not going to realize any of this," said Elias Konstantopolous, 74, a retired electrician in Baltimore, one of about 50 Americans and Europeans who have been using the device in clinical trials for several years.
- He said it helps him differentiate curbs from asphalt roads and detect contours, but not details, of cars, trees and people.
- "When you don't have nothing, this is something," Mr. Konstantopolous said. "It's a lot." (from smh.com.au)
